Synthesis, Molecular Docking, and In Vitro Activity Test of Thioxanthenol and Nitrothioxanthone Derivatives As Anticancer Agents
Abstract
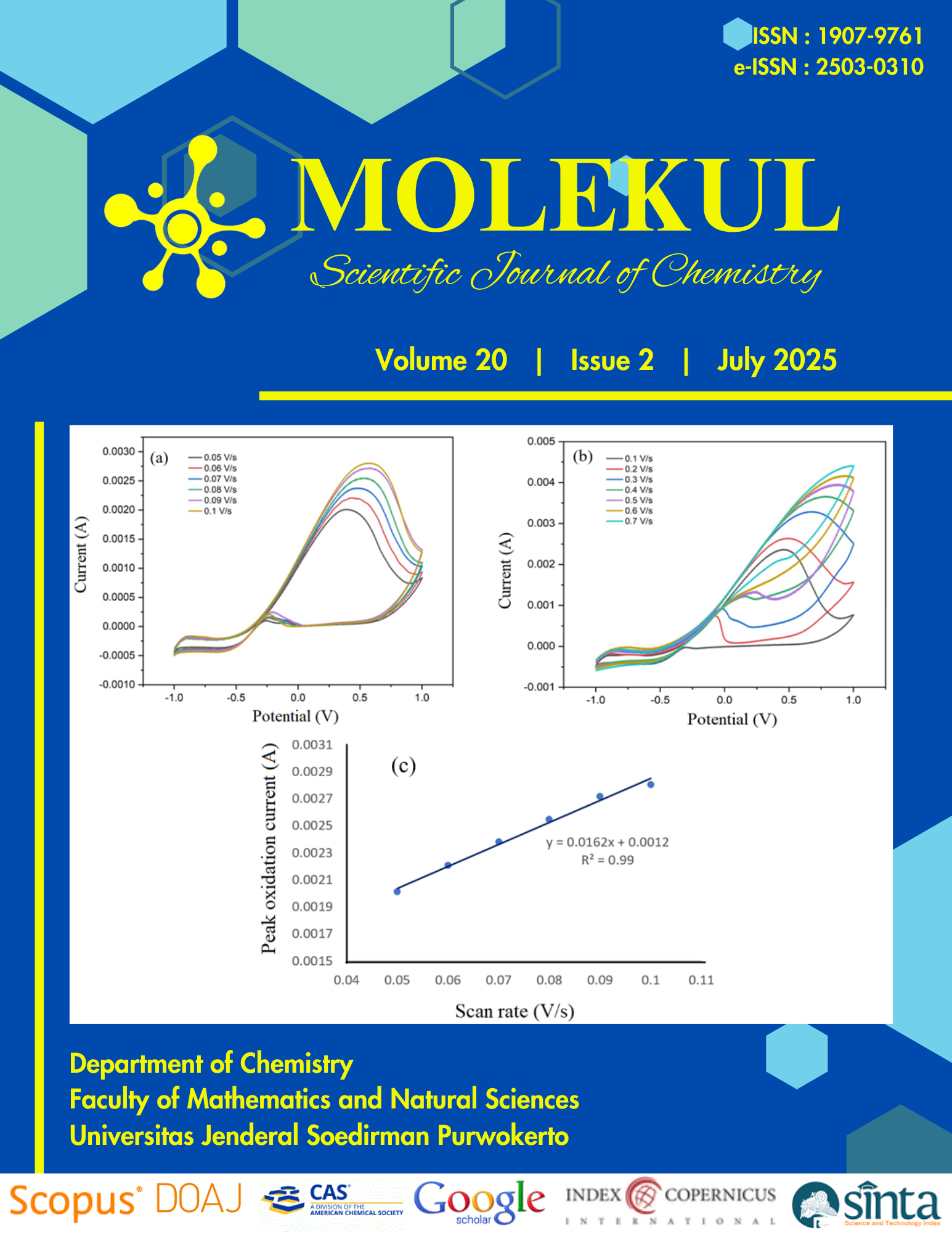
ABSTRACT. This research aimed to compare, synthesise, study molecular docking, and test the anticancer activity of thioxanthenol, 1-hydroxythioxanthone, 4-nitrothioxanthone, and 2-nitrothioxanthone compounds through in silico and in vitro assays, highlighting their selective cytotoxicity and potential as novel anticancer scaffolds. These four compounds were obtained through reduction and nitration reactions of the thioxanthone. Thioxanthenol compound was obtained through the reduction of thioxanthone using sodium borohydride. The 1-hydroxythioxanthone, 4-nitrothioxanthone, and 2-nitrothioxanthone compounds were obtained from the nitration of thioxanthone compounds. The compounds were characterised using FTIR, GC-MS, 1H-NMR, and 13C-NMR. In vitro cytotoxicity tests were performed using microtetrazolium (MTT) assays against T47D, WiDr, and Hela cancer cell lines and the Vero cell line as normal cells. The molecular docking process was studied to determine the in silico activity of the compounds with protein targets. The reduction reaction produced the thioxanthenol compound as a yellowish-white solid in 40.63% yield. The nitration reaction produced 1-hydroxythioxanthone, 4-nitrothioxanthone, and 2-nitrothioxanthone compounds as light-yellow solids in 33.54%; 29.27%; and 31.71% yield, respectively. The synthesized compounds demonstrated selective anticancer activity against certain cancer cells. Thioxanthenol compound showed an IC50 value of 17.46 µg mL-1 on the WiDr cell line and nitrotioxanthone compound showed an IC50 value of 6.05 µg mL-1 on the T47D cell line. Molecular docking showed that the thioxanthone derivatives might act as the anticancer agent through inhibition of epidermal growth factor receptor (EGFR), P-glycoprotein, and Erα functions.
Keywords: anticancer, nitrothioxanthone, thioxanthenol, thioxanthone
Authors agree with the statements below:
- Authors automatically transfer the copyright to the MOLEKUL journal and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
- Authors are able to enter into separate permission for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.